Abstract
Preterm infants are at risk of increased trans-epidermal water loss and infections due to epidermal immaturity. The emollient and anti-infective properties of coconut oil make it a potentially beneficial topical agent for this population. We aimed to systematically review randomised trials assessing the effects of topical coconut oil in preterm infants. Medline, EMBASE, Cochrane Central Register of Controlled Trials and CINAHL were searched. Seven trials (n = 727 infants) were included. The majority of trials included relatively mature infants (gestation > 32 weeks, birth weight > 1200 g). The duration of intervention (5–31 days) and outcomes of interest varied among included studies. Meta-analysis using random effects model found significantly lower incidence of hospital-acquired blood stream infections (HABSI) in the coconut oil group (11/164 vs 32/166; relative risk 0.35, 95% confidence interval 0.18, 0.67, p = 0.001; I2 = 0%, two RCTs). Overall, infants in the coconut oil group had decreased water loss, decreased infection rates, better growth and skin condition. There were no significant adverse effects associated with coconut oil application. The overall quality of evidence was considered moderate for the outcome of HABSI and low for the outcome of physical growth based on GRADE guidelines.
Conclusion: Topical coconut oil application to the skin may be beneficial in preterm infants, but the quality of evidence is low to moderate. Adequately powered randomised controlled trials, especially in very preterm (< 32 weeks) and extremely preterm (< 28 weeks) infants, are needed.
What is Known: • Coconut oil has been used traditionally for topical application in terms of infants in Asian countries | |
What is New: • This systematic review found that topical application of coconut oil may reduce the risk of infection and improve weight gain and skin condition in preterm infants. However, the quality of evidence was considered to be moderate to low based on GRADE guidelines. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mature epidermis acts as a physical barrier to minimise excessive loss of water, prevents the absorption of noxious substances and protects against micro-organisms [4]. An absent or immature stratum corneum results in very high trans-epidermal water loss (TEWL) in extremely preterm infants [4]. Development of the foetal skin is dramatically hastened by preterm birth and the epidermis is histologically similar to that of term infant by 2 to 3 weeks after birth [4, 29, 30]. The vernix, which starts developing during the last trimester, functions as an emollient to maintain hydration, facilitates the formation of acid mantle, essential for microbial homeostasis and first-line host defence and has anti-oxidant properties.
As very preterm infants have an immature stratum corneum and lack vernix, emollients have the potential to enhance the skin barrier function [22]. Fatty acids from topically applied oils can actively be taken up by keratinocytes, making them available for dermal metabolism, augmenting its structure and function [7]. Emollients may also act through activation of a nuclear hormone receptor that in turn activates genes involved in skin development [7].
Various emollients including natural oils derived from sunflower seeds, coconuts, soya bean, olives and almonds as well as mineral oil–based products such as petroleum jelly, Aquaphor© and Eucerin© cream have been evaluated in randomised controlled trials (RCT) in preterm neonates with variable results [6, 8, 25]. Of these, coconut oil has shown superior potential to improve clinical outcomes in view of its high content of medium-chain fatty acids, especially lauric acid [14, 20]. Lauric acid and its ester, monolaurin, naturally occur on the human epithelial surfaces and both have anti-inflammatory and antimicrobial properties, including against neonatal pathogens, particularly staphylococcal species [2, 3, 10, 16, 19, 21]. In contrast, animal studies have shown that soya bean, mustard and olive oil can significantly delay the recovery of epidermal barrier function after an injury [24]. To our knowledge, there are no systematic reviews specifically on coconut oil application in preterm infants. Hence, we conducted a systematic review to evaluate the efficacy and safety of topically applied coconut oil in preterm infants.
Methods
Guidelines from the Cochrane handbook (http://handbook.cochrane.org/), the PRISMA statement were followed for undertaking and reporting this systematic review [15]. Ethics approval was not required.
Eligibility criteria
-
1.
Types of studies: Only RCTs were eligible for inclusion.
-
2.
Types of participants: Preterm (< 37 weeks) or low birth weight (< 2500 g) infants.
-
3.
Intervention and comparison: Coconut oil application to the skin vs standard management.
-
4.
Outcomes: Hospital-acquired blood stream infections (HABSI), physical growth (z scores for weight, length and head circumference), fatty acid levels in blood, skin integrity, TEWL.
Search strategy
The databases PubMed (www.ncbi.nlm.nih.gov, 1966 to September 2018), EMBASE via Ovid (http://ovidsp.tx.ovid.com, 1980 to September 2018), Cochrane Central Register of Controlled Trials (www.thecochranelibrary.com, through September 2018) and Emcare via OVID (http://ovidsp.tx.ovid.com, 1980 to September 2018) were searched. Grey literature was searched using the national technical information services (http://www.ntis.gov/), Open Grey (http://www.opengrey.eu/) and Trove (http://trove.nla.gov.au/). The reference lists of eligible studies and review articles were searched to identify additional studies. Two reviewers conducted the literature search independently. No language restriction was applied.
The following terms were used for searching on PubMed: ((((preterm infants) OR preterm infant) OR (“Infant, Extremely Premature” OR “Infant, Extremely Low Birth Weight”[Mesh] OR “Infant, Very Low Birth Weight”[Mesh] OR “Infant, Premature, Diseases”[Mesh] OR “Infant, Low Birth Weight”[Mesh] OR “Infant, Premature”[Mesh]))) AND (coconut oil) OR ((((“Coconut Oil”[Mesh]) OR “Administration, Topical”[Mesh]) OR massage) OR “Massage”[Mesh]). PubMed was also searched using relevant key words. Other databases were searched using similar terminologies. Cross references of relevant studies were read to identify any additional studies.
Study selection
Abstracts of the citations obtained from the initial broad search were read independently by two reviewers (Sameer Pupala, Shripada Rao) to identify potentially eligible studies. Full-text articles of these studies were obtained and assessed for eligibility by two reviewers independently using the predefined eligibility criteria. Differences in opinion were resolved by discussion between all reviewers to reach consensus. Multiple publications from the same cohort of preterm infants were included only if any additional information was available; otherwise, they were considered to be duplicates, and information was used only once.
Data extraction
Two reviewers (Sameer Pupala and Shripada Rao) extracted the data independently using a data collection form designed for this review. Discrepancies during the data extraction process were resolved by discussion among all authors and consensus was reached.
Assessment of risk of bias
This was assessed using the Cochrane “Risk of Bias Assessment Tool” for RCTs [12].
Data synthesis
Where appropriate, meta-analysis using random effects model was conducted using the RevMan 5.3 software to pool the data (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014). Where data was not available in a format to enable pooling, a descriptive synthesis was performed.
GRADE evidence
The key information concerning the quality of evidence, based on the (a) sample size for clinically important outcomes, (b) magnitude and precision of the treatment effect of the intervention, (c) risk of bias, (d) directness of evidence and (e) consistency of results (statistical heterogeneity), was planned to be assessed as per GRADE guidelines (Grades of Recommendation, Assessment, Development and Evaluation) [11]. All authors discussed and agreed with the GRADE allocation.
Results
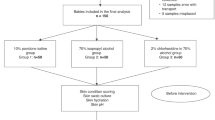
Initial broad search yielded 710 citations. After excluding duplicates and non-relevant studies, seven were considered as eligible and hence included in the review. The final sample size was n = 727 infants from seven RCTs. Figure 1 gives details of the study selection process. The characteristics of included studies and risk of bias assessment are given in Tables 1 and 2.
-
1.
Solanki et al. [27] randomised 72 preterm infants to three treatment groups: (i) safflower oil (n = 27), (ii) coconut oil (n = 25) and (iii) no oil controls (n = 20). They also included 46 term infants. Five millilitres of the study oil was massaged for 10 min four times a day for 5 days, starting at a mean (SD) age of 1.54 (0.7), 1.53 (0.7) and 1.56 (0.8) days, respectively. The mean (SD) gestational age and birth weight were as follows. Safflower oil: 35.2 weeks (2.8), 2078 g (561); coconut oil: 35.2 weeks (2.0), 1938.3 g (588.7); controls: 35.2 weeks (3.1), 1991.2 g (590.2). The primary outcome was transcutaneous absorption of fatty acids. Blood samples were analysed for triglycerides and fatty acid profiles using gas chromatography. Post-massage, triglyceride and fatty acid levels increased significantly in both the oil groups and also in controls. However, the proportionate rise was significantly higher in the oil groups as compared with controls. Fatty acid profiles showed significant proportionate increase as compared with the control group in mean (SD) linoleic acid levels (< 37 weeks 1.36 μg/dL (1.0–7.7) vs 1.11 (1.0–3.33); > 37 weeks 5.96 μg/dL (1.01–6.36) vs 1.0 (0.90–3.66) p < 0.05) in the safflower oil group and in saturated fat levels (< 37 weeks 3.03 μg/dL (0.79.0–7.88) vs 1.73 (1.0–8.07); > 37 weeks 8.44 (1.76–28.50) vs 0.9 (0.38–5.58) p < 0.01) in the coconut oil group. No side effects of the oil massage were noted. The authors concluded that topically applied oil can be absorbed in infants and is probably available for nutritional purposes.
-
2.
Sankaranarayanan et al. [26] randomised 112 preterm infants to receive massage with coconut oil (n = 38), mineral oil (n = 37) or placebo (n = 37). They also included 112 term infants, but provided results separately for preterm and term infants. Oil massage was given by trained staff from day 2 of life until discharge, and thereafter by the mother until 1 month of age. The oil was massaged for 5 min, four times a day from day 2 of life until discharge and thereafter at home until 31 days of age. Among the preterm infants, the mean (SD) gestation (coconut oil: 34.8 weeks (1.2), mineral oil: 34.7 (1.1), control: 34.9 (1.1)) and birth weight (coconut oil: 1792 (149), mineral oil: 1758.3 g (79.91), placebo: 1789 (182.90)) were comparable. The primary outcome was weight gain velocity over the first 31 days of life. The coconut oil group showed significantly higher weight gain velocity compared with the mineral oil and placebo group.
Preterm infants receiving coconut oil massage also showed a greater length gain velocity (mean 0.6 (SD 0.12), p < 0.05) compared with placebo (0.56 (0.16)). Mild rash not requiring discontinuation of treatment was noted in six preterm babies (2 in each arm) and eight term babies (3 each in oil groups and two in placebo). As this study required the mothers to continue intervention at home, compliance with treatment could not be monitored.
-
3.
Jansi et al. [13] randomised 64 low birth weight infants. Intervention group were applied 5 mL/kg/day of coconut oil twice a day for 5 days and controls were given routine care. The application was initiated between day 1 and day 15. The mean (SD) gestation and birth weight were 36 (2.2) vs 35.2 (2.2) weeks and 2.1 (0.4) vs 1.9 (0.4) kg, respectively. There was no change in weight. The neurobehavioural response, assessed by the neonatal intensive care unit network neurobehavioural scale prior to and after completion of the 5 days of the study intervention, showed significant improvement (p < 0.05) in mean (SD) scores for habituation (day 1: 3.9 (0.39), day 5: 5.2 (0.49) vs day 1: 4.0 (0.05) and day 5: 4.05 (0.61)) and attention (day 1: 3.49 (0.5), day 5: 5.5 (2.72) vs day 1: 3.1 (0.4), day 5: 4.5 (1.0)).
-
4.
Saeedi et al. [23] randomised 75 preterm infants < 37 weeks to one of the following groups: coconut oil massage (n = 25), massage without oil (n = 25), routine care (n = 25) from day 2 to 10. The mean (SD) birth weights were 1738.8 g (495.2), 1779.5 (472.4) and 1424.8 (325.3), respectively (p > 0.05). The mean (SD) gestation were 32.8 weeks (3.1), 33 (2.3), 31.3 (2.2), respectively. Four millilitres of Coconut oil was applied for 5 min four times a day for 7 days. The primary outcome was weight gain after 7 days of intervention. The coconut oil group showed significant weight gain (mean 212.4 g (SD 240.8), p < 0.002) compared with massage only (7.39 (96.86)) and the control group (224.4 (28)). No adverse events were reported.
-
5.
Salam et al. [25] evaluated the efficacy of topical coconut oil application in preterm infants (26–37 weeks). Five millilitres per kilogram of coconut oil was applied by nurses over 2–3 min twice daily from birth until discharge and continued subsequently by the mothers at home until day 28. A total of 258 preterm infants were randomised to receive coconut oil or standard care. The mean (SD) gestational age and weights were 33.9 weeks (2.6) vs 34.4 (2.6) and 2114 g (499.7) vs 2249.5 (569.0) respectively. The primary outcome was clinically suspected and blood culture-proven LOS, which occurred in 14% of infants in the intervention group vs 23% in the control group. After adjusting for gestational age, birth weight and duration of intubation and hospitalisation, the HR for late-onset sepsis was significantly higher in the control group compared with the coconut oil group (HR 6.0, 95% CI 2.3 to 16). The rates of LOS in the control and coconut oil groups were 219.1 and 39.5/1000 patient days, respectively. Within the secondary outcomes, mean weight gain was 11.3 g/day higher (95% CI 8.1–14.6, p < 0.0001) and the skin condition was significantly better in the intervention group. There were no significant differences in the duration of hospitalisation or neonatal mortality. No adverse effects were observed.
-
6.
Nangia et al. [17] randomised 74 very low birth weight infants at 12 h of age to coconut oil application (n = 37) or control (n = 37) groups. The mean (SD) gestational age and birth weight of infants included in the trial were 31.89 weeks (2.21) vs 31 (2.45) and 1213.5 g (214.4) vs 1163.7 g (208.9), respectively. Four millilitres of coconut oil was applied twice daily without massage during the first week of life, while the control group received standard care. The primary outcome was TEWL and secondary outcomes were skin-condition scores and skin colonisation. TEWL was measured twice daily for 7 days using an evaporimeter. On day 7, skin swabs were obtained for bacterial culture and skin condition was assessed using a validated score. TEWL was significantly reduced in the coconut oil group at all time points with a mean difference of 6.80 g/m2/h (95% CI 3.48 to 10.15; p < 0.01). The proportion of infants with good skin condition (i.e. score of 1) was significantly higher in the oil group (54.5% vs 10.81%). Skin swabs were positive for microbial growth in 20% of oil group as compared with 60% of controls.
-
7.
Strunk et al. [28] randomised 72 very preterm infants (< 30 weeks). Infants in the intervention group received topical coconut oil (5 mL/kg) twice daily for 21 days, starting within 24 h of birth, while the control group received routine care. The median (IQR) gestations were 27.9 weeks (26.3–29.3) and 27.9 (25.4–29.1), respectively. The median (IQR) birth weights were 1070 g (879–1202) and 950 (726–1155), respectively. The primary outcomes were safety, feasibility and efficacy in terms of improvement in skin integrity scores. The coconut oil group did not have any adverse events and all of the enrolled participants completed the trial. Skin integrity scores were maintained in the coconut oil group throughout the intervention period but deteriorated in the control group from a median (IQR) of 3 (3–4) on day 1 to 4 (4–4) on day 21 in the control group (p = 0.01).
Meta-analysis
Pooling of data using random effects model found significantly lower incidence of hospital-acquired blood stream infections in the coconut oil group (11/164 vs 32/166; relative risk 0.35, 95% confidence interval 0.18, 0.67, p = 0.001; I2 = 0%, two RCTs) (Fig. 2). Meta-analysis could not be done for the outcome of physical growth due to the lack of data from the included studies in a format suitable for pooling.
Risk of bias in the included studies
Majority of the studies had low risk of bias for the domains of random sequence generation and allocation concealment; however, blinding was not possible due to the nature of the intervention. Details of assessment are given in Table 2.
GRADE evidence
Quality of evidence for the outcome of HABSI was downgraded by two levels in view of the issues with risk of bias and the inability to assess publication bias. On the contrary, the quality was upgraded by one level in view of the large effect size (RR < 0.5). Hence, the final quality of evidence was deemed moderate (Table 3). For the outcome of physical growth, the quality of evidence was deemed low because of the following reasons: (a) final effect size could not be estimated due to the lack of data and (b) statistical heterogeneity could not be calculated.
Discussion
This systematic review of 727 preterm infants from seven RCTs found topical application of coconut oil to be safe and probably beneficial in reducing the risk of HABSI, improving the skin integrity and nutrition. However, the quality of evidence based on GRADE guidelines was deemed only moderate for the outcome of HABSI and low for the outcome of physical growth.
There is evidence from animal research that topical application of coconut oil improves skin healing [18]. In Sprague-Dawley rats, experimental wounds were treated with coconut oil for 10 days and compared with no oil application. Coconut oil–treated wounds healed significantly faster, lipid peroxide levels were lower and neovascularisation was increased in coconut oil–treated wounds [18].
TEWL is a major problem in extremely preterm infants in the first week of life [4]. Coconut oil application has the potential to overcome this problem. Further, coconut oil application has been shown to be of some benefit in children with atopic dermatitis and xerosis and common childhood conditions associated with high TEWL [1, 9] and it reduces staphylococcal colonisation in adult atopic dermatitis.
Preterm infants in the intensive care setting undergo numerous procedures that compromise their skin integrity. These interventions are most commonly performed early in life when the skin is most vulnerable and therefore, topical coconut oil may be most beneficial if applied early.
A recent Cochrane review evaluated the use of topical emollients in preterm infants. A total of 3089 infants from 19 trials conducted in a variety of settings including low-, middle- and high-income countries were included. Application of topical creams or ointments did not reduce the incidence of invasive infections. Meta-analyses for trials using topical vegetable oils showed no difference in the incidence of invasive infection or mortality [5, 6]. Another meta-analysis of 8 RCTs conducted predominantly in low- and middle-income countries found that topical vegetable oils significantly reduced mortality and LOS and enhanced growth in preterm infants [24]. Such contradicting conclusions suggest uncertainty in this area and that effects might differ by type of oil, study population and environment. This highlights the need for further high-quality studies.
To our knowledge, this is the first systematic review evaluating the efficacy and safety of topical application of coconut oil in preterm infants. The strength of our review was detailed search strategy and stringent methodology following the Cochrane and GRADE guidelines. The main limitation was the low sample size, heterogeneous nature of reporting of physical growth and variable duration of follow-up in the included studies. All studies except one were conducted in low- and middle-income settings, which prevent the generalisability of results. Although the majority of RCTs included moderate to late preterm infants (mean gestation > 32 weeks, birth weight > 1200 g), it was also found to be safe and effective in maintaining skin integrity in extremely low gestational age infants in one of the trials [26].
Even though our systematic review found that topical application of coconut oil may be beneficial, it is important to note that the overall quality of evidence was low to moderate. Hence, firm recommendations cannot be made supporting the universal use of topical application of coconut oil in preterm infants. However, the LMIC study conducted by Salam et al. was adequately powered to answer clinically relevant questions. It showed significant reduction in the incidence of LOS in the coconut oil group. Hence, neonatal units in LMICs could consider routine application of coconut oil for their preterm infants. It is also reasonable for individual countries to conduct RCTs in their preterm infant population using a protocol similar to that of Salam et al. On the contrary, adequately powered RCTs, especially in the very preterm (< 32 weeks) and extremely preterm (< 28 weeks) infants, are needed to determine the safety and efficacy of this intervention.
Abbreviations
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- IQR:
-
Inter quartile range
- LOS:
-
Late-onset sepsis
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT:
-
Randomised controlled trial
- SD:
-
Standard deviation
- TEWL:
-
Trans-epidermal water loss
References
Agero AL, Verallo-Rowell VM (2004) A randomized double-blind controlled trial comparing extra virgin coconut oil with mineral oil as a moisturizer for mild to moderate xerosis. Dermatitis 15:109–116
Batovska DI, Todorova IT, Tsvetkova IV, Najdenski HM (2009) Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: individual effects and synergistic relationships. Pol J Microbiol 58:43–47
Carpo BG, Verallo-Rowell VM, Kabara J (2007) Novel antibacterial activity of monolaurin compared with conventional antibiotics against organisms from skin infections: an in vitro study. J Drugs Dermatol 6:991–998
Cartlidge P (2000) The epidermal barrier. Semin Neonatol 5:273–280
Cleminson J, McGuire W (2015) Topical emollient for prevention of infection in preterm infants: a systematic review. Lancet 385(Suppl 1):S31
Cleminson J, McGuire W (2016) Topical emollient for preventing infection in preterm infants. Cochrane Database Syst Rev CD001150
Darmstadt GL, Mao-Qiang M, Chi E, Saha SK, Ziboh VA, Black RE, Santosham M, Elias PM (2002) Impact of topical oils on the skin barrier: possible implications for neonatal health in developing countries. Acta Paediatr 91:546–554
Edwards WH, Conner JM, Soll RF, Vermont Oxford Network Neonatal Skin Care Study G (2004) The effect of prophylactic ointment therapy on nosocomial sepsis rates and skin integrity in infants with birth weights of 501 to 1000 g. Pediatrics 113:1195–1203
Evangelista MT, Abad-Casintahan F, Lopez-Villafuerte L (2014) The effect of topical virgin coconut oil on SCORAD index, transepidermal water loss, and skin capacitance in mild to moderate pediatric atopic dermatitis: a randomized, double-blind, clinical trial. Int J Dermatol 53:100–108
Fischer CL, Blanchette DR, Brogden KA, Dawson DV, Drake DR, Hill JR, Wertz PW (2014) The roles of cutaneous lipids in host defense. Biochim Biophys Acta 1841:319–322
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schunemann HJ (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394
Higgins JP, Green S (eds) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration
Jansi LBR (2008) Effect of oil massage on changes in weight and neurobehavioural response of low birth weight babies. Nurs J India 99:256
Lomangino K (2012) Coconut oil and health: assessing the evidence. Clin Nutr Insight 38:1–4
Moher D, Liberati A, Tetzlaff J, Altman DG, The PG (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med 6:e1000097
Nakatsuji T, Kao MC, Fang JY, Zouboulis CC, Zhang L, Gallo RL, Huang CM (2009) Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Investig Dermatol 129:2480–2488
Nangia S, Paul VK, Deorari AK, Sreenivas V, Agarwal R, Chawla D (2015) Topical oil application and trans-epidermal water loss in preterm very low birth weight infants-a randomized trial. J Trop Pediatr 61:414–420
Nevin KG, Rajamohan T (2010) Effect of topical application of virgin coconut oil on skin components and antioxidant status during dermal wound healing in young rats. Skin Pharmacol Physiol 23:290–297
Ogbolu DO, Oni AA, Daini OA, Oloko AP (2007) In vitro antimicrobial properties of coconut oil on Candida species in Ibadan, Nigeria. J Med Food 10:384–387
Pehowich DJ, Gomes AV, Barnes JA (2000) Fatty acid composition and possible health effects of coconut constituents. West Indian Med J 49:128–133
Preuss HG, Echard B, Enig M, Brook I, Elliott TB (2005) Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram-positive and gram-negative bacteria. Mol Cell Biochem 272:29–34
Rutter N (1996) The immature skin. Eur J Pediatr 155(Suppl 2):S18–S20
Saeedi R, Gholami M, Dinparvar S, Kabirian M (2011) Transcutaneous feeding: the effect of massage with coconut oil on weight gaining in preterm newborns. Iran Red Crescent Med J 13:666–669
Salam RA, Das JK, Darmstadt GL, Bhutta ZA (2013) Emollient therapy for preterm newborn infants--evidence from the developing world. BMC Public Health 13(Suppl 3):S31
Salam RA, Darmstadt GL, Bhutta ZA (2015) Effect of emollient therapy on clinical outcomes in preterm neonates in Pakistan: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 100:F210–F215
Sankaranarayanan K, Mondkar JA, Chauhan MM, Mascarenhas BM, Mainkar AR, Salvi RY (2005) Oil massage in neonates: an open randomized controlled study of coconut versus mineral oil. Indian Pediatr 42:877–884
Solanki K, Matnani M, Kale M, Joshi K, Bavdekar A, Bhave S, Pandit A (2005) Transcutaneous absorption of topically massaged oil in neonates. Indian Pediatr 42:998–1005
Strunk T, Pupala S, Hibbert J, Doherty D, Patole S (2018) Topical coconut oil in very preterm infants: an open-label randomised controlled trial. Neonatology 113:146–151
Visscher M, Narendran V (2014) The ontogeny of skin. Adv Wound Care (New Rochelle) 3:291–303
Visscher MO, Narendran V, Pickens WL, LaRuffa AA, Meinzen-Derr J, Allen K, Hoath SB (2005) Vernix caseosa in neonatal adaptation. J Perinatol 25:440–446
Author information
Authors and Affiliations
Contributions
Sameer Pupala: Concept, design, independent literature search selection of studies for inclusion, extraction and interpretation of the data, assessing the risk of bias of included studies, GRADE evidence, writing the first and final drafts of the manuscript; Shripada Rao: Independent literature search, selection of studies for inclusion, data extraction, meta-analysis to generate forest plots, GRADE evidence, helping with the first and final draft of the manuscript; Tobias Strunk: Concept, design, interpretation of the data, GRADE evidence, critical review of the first and the final draft of the manuscript. Sanjay Patole: Concept, design, interpretation of the data, GRADE evidence, critical review of the first and the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Not applicable since it was a systematic review.
Additional information
Communicated by Patrick Van Reempts
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pupala, S.S., Rao, S., Strunk, T. et al. Topical application of coconut oil to the skin of preterm infants: a systematic review. Eur J Pediatr 178, 1317–1324 (2019). https://doi.org/10.1007/s00431-019-03407-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03407-7